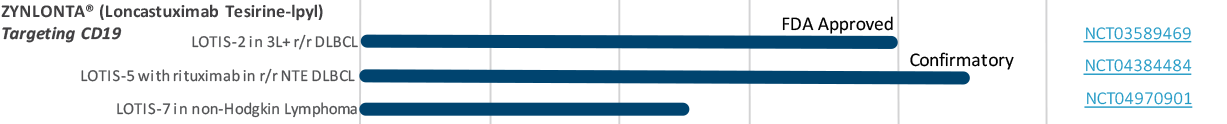Advancing scientific knowledge

Our Company
Our Science

Our Pipeline
Recent Data
Explore the latest research from our scientists
Initial Results From LOTIS-7: A Phase 1b Study of Loncastuximab Tesirine Plus Glofitamab in Patients With Relapsed/Refractory (R/R) Diffuse Large B-Cell Lymphoma (DLBCL)
EHA, 2025
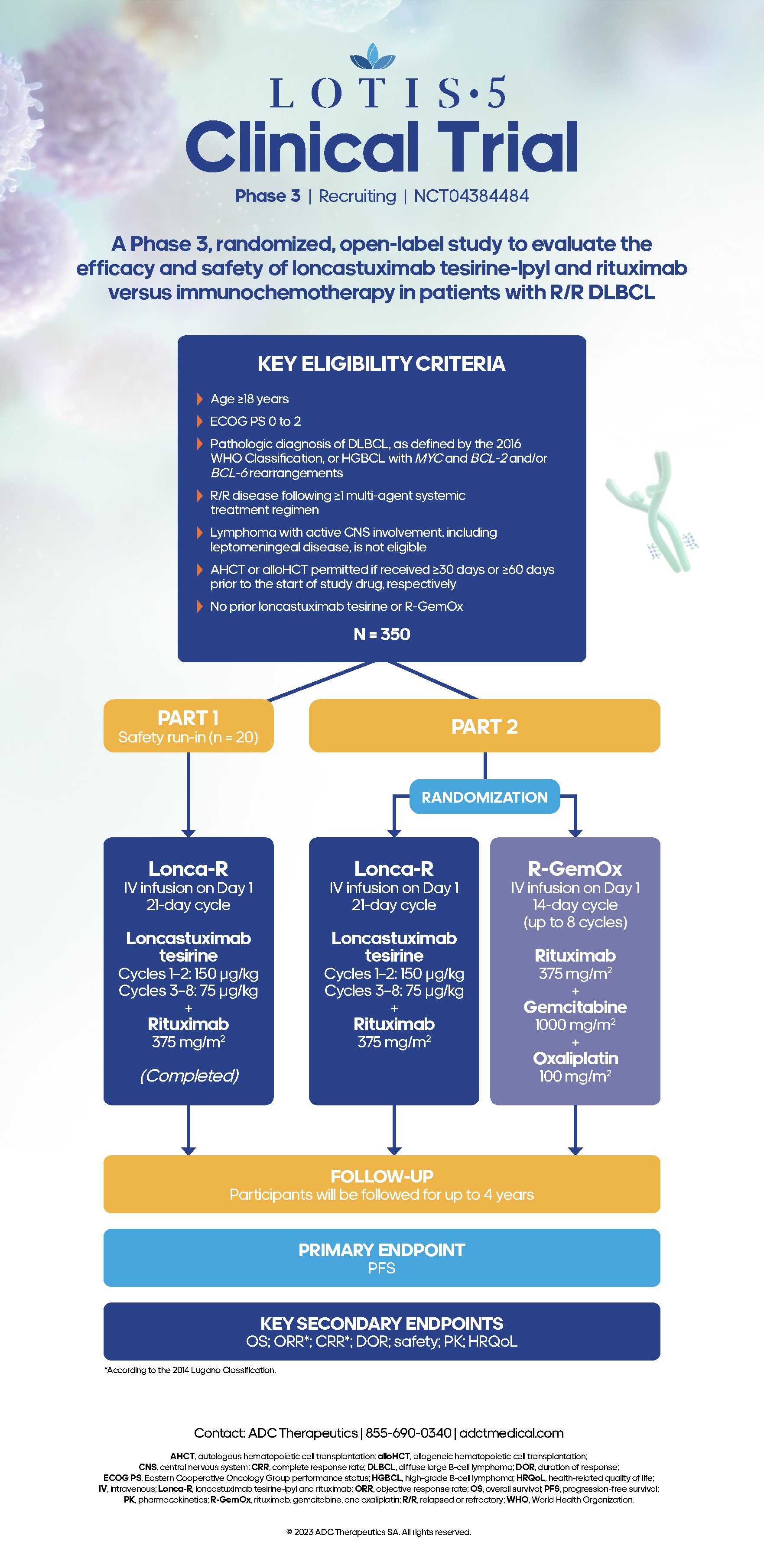
Updated Safety Run-In Results From LOTIS-5: A Phase 3, Randomized Trial of Loncastuximab Tesirine With Rituximab Versus Immunochemotherapy in Patients With R/R DLBCL/HGBL
EHA, 2025
Updated analysis of a phase 2 multicenter study of the loncastuximab in relapsed/refractory marginal zone lymphoma demonstrates high rate of complete responses
ICML, 2025
Preclinical investigation of ADCT-242, a novel exatecan-based antibody drug conjugate targeting Claudin-6, as single agent or in combination in ovarian and non-small lung cancer models
AACR, 2025
Preclinical development of ADCT-241, a novel exatecan-based antibody-drug conjugate targeting PSMA for the treatment of prostate cancer
AACR, 2025
HuB14-VA-PL2202, a novel antibody-drug conjugate targeting ASCT2, a novel ADC target over-expressed in both solid and hematological cancers
AACR, 2025
Loncastuximab Tesirine with Rituximab Induces Robust and Durable Complete Metabolic Responses in High-Risk Relapsed/Refractory Follicular Lymphoma
ASH, 2024
Independent Investigator-Initiated Trial
Limited Duration Loncastuximab Tesirine Induces a High Rate of Complete Responses in Patients with R/R Marginal Zone Lymphoma – Report of First Planned Interim Futility Analysis
ASH, 2024
Independent Investigator-Initiated Trial
Loncastuximab Tesirine in Combination with Venetoclax Is Safe and Shows Efficacy in Patients with Relapsed/Refractory Non Hodgkin Lymphoma
ASH, 2024
Independent Investigator-Initiated Trial
A phase II trial of loncastuximab tesirine in patients with previously treated Waldenström macroglobulinemia
ASH, 2024
Independent Investigator-Initiated Trial
Phase 1b Open-Label Study of Loncastuximab Tesirine in Combination With Other Anticancer Agents in Patients With Relapsed/Refractory B-cell Non-Hodgkin Lymphoma (LOTIS-7)
SOHO, 2024
LOTIS-5, an Ongoing, Phase 3, Randomized Study of Loncastuximab Tesirine With Rituximab (Lonca-R) Versus Immunochemotherapy in Patients With R/R DLBCL
ASCO, 2024
Quantitative systems pharmacology (QSP) model to predict combination activity of CD19-targeted antibody drug conjugate (loncastuximab tesirine) co-dosed with a CD20/CD3 T-cell bispecific (epcoritamab) in patients with diffuse large B-cell lymphoma (DLBCL)
ASCO, 2024
Quantitative systems pharmacology modeling of loncastuximab tesirine combined with mosunetuzumab and glofitamab helps guide dosing for patients with DLBCL
AACR, 2024
Updated Results of the Safety Run-In of the Phase 3 LOTIS-5 Trial: Novel Combination of Loncastuximab Tesirine With Rituximab (Lonca-R) Versus Immunochemotherapy in Patients With R/R DLBCL
SOHO, 2023




MedConnect
Real-time answers to your pressing questions
Connect live with
Medical Affairs
Our Company
Driven by our dedication to improving outcomes in difficult-to-treat cancers, we are leading the development and commercialization of next-generation antibody drug conjugates (ADCs) with highly potent and targeted pyrrolobenzodiazepine (PBD) dimer technology.
Read and download our brochure
Our Science
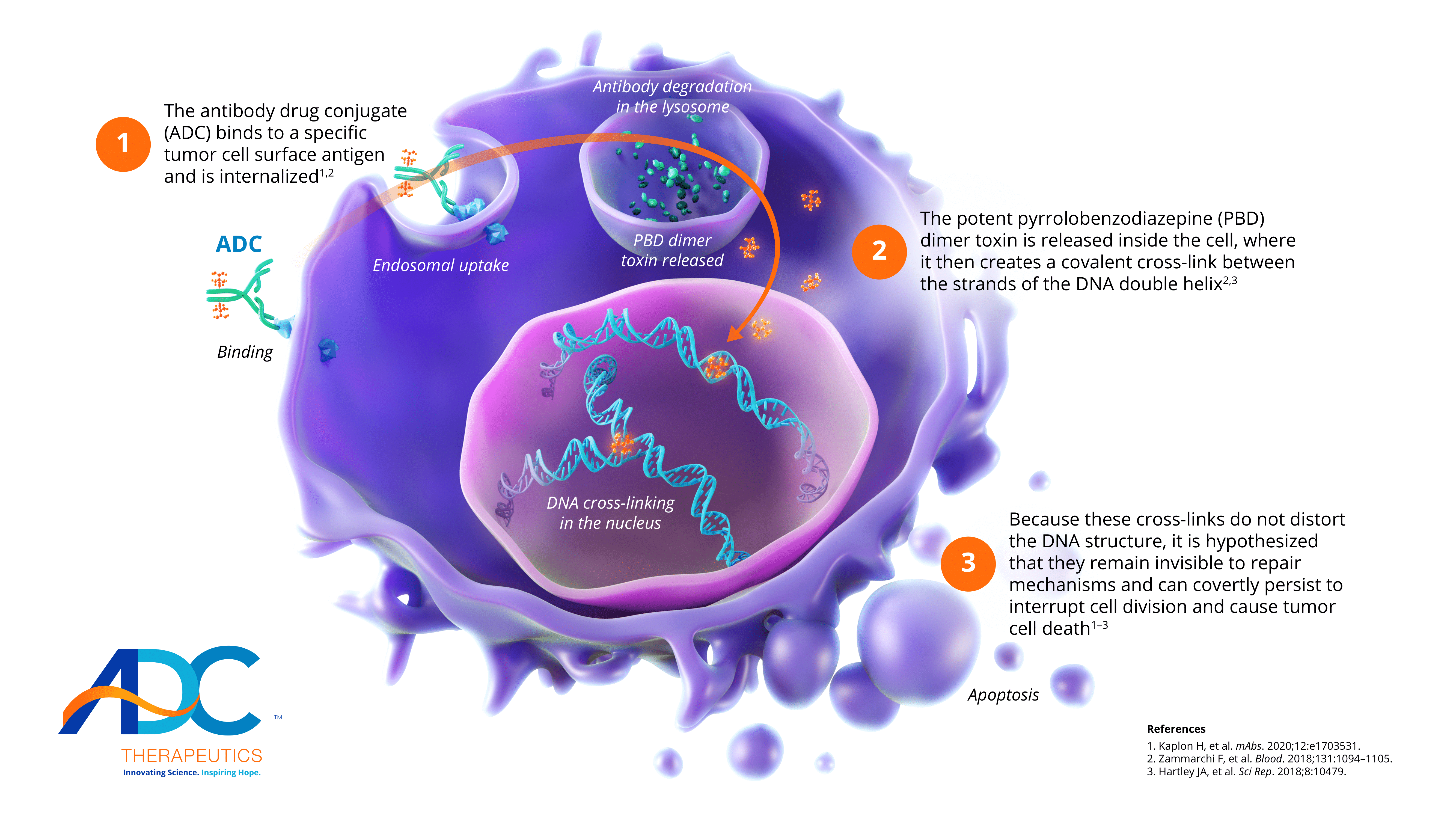
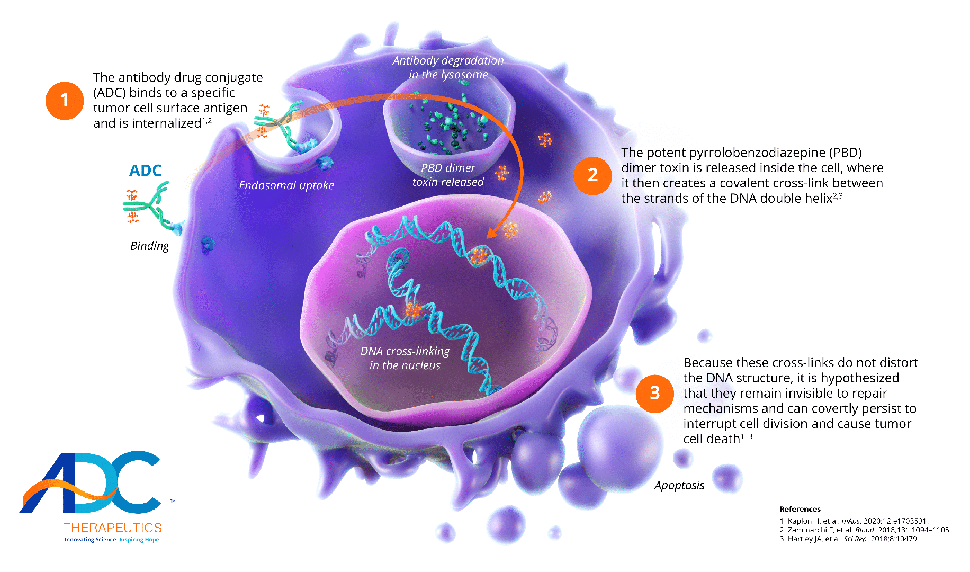
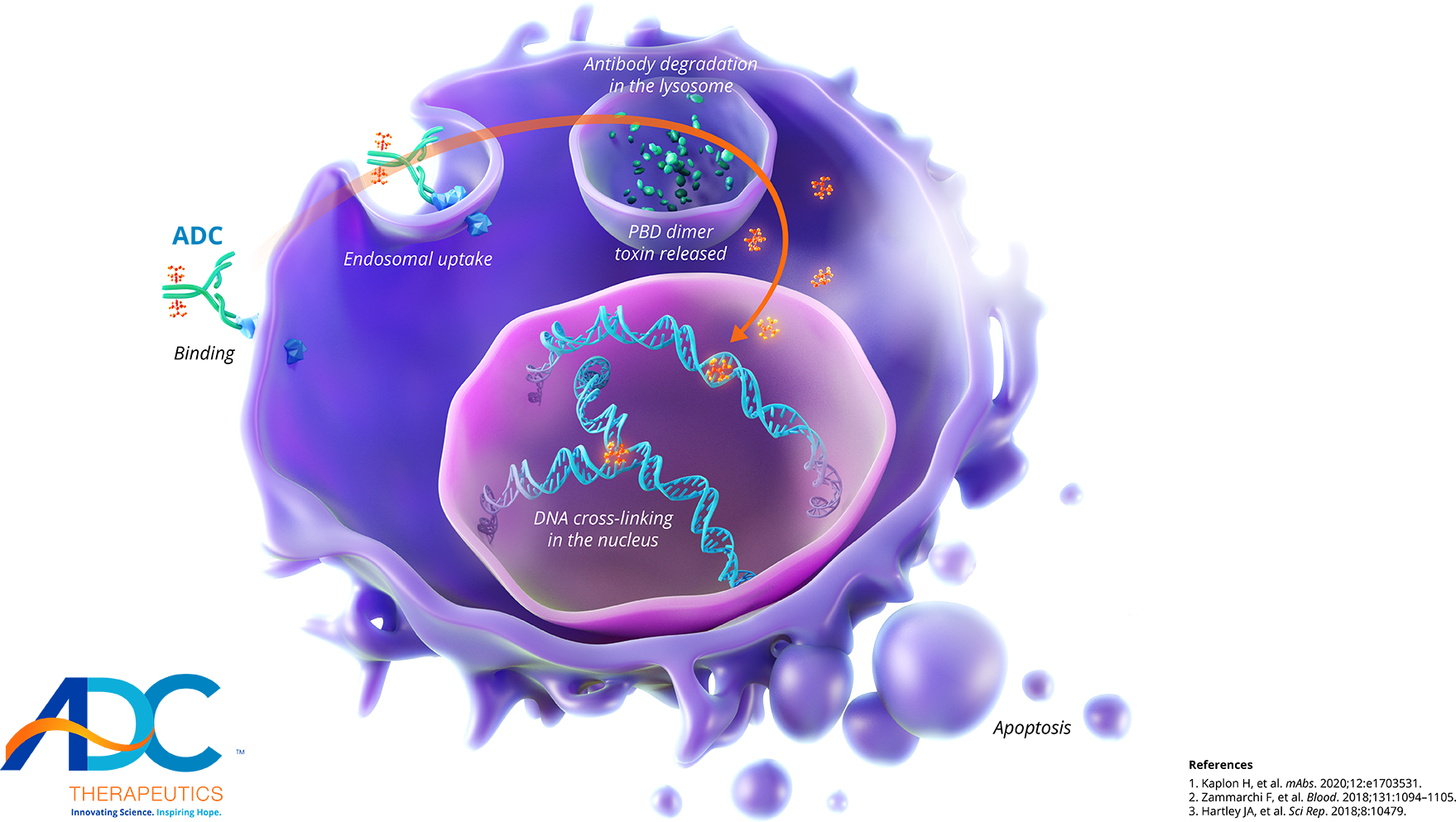
Advancing antibody drug conjugates with a novel class of PBD dimersADC Therapeutics’ proprietary ADCs are highly targeted drug constructs that combine monoclonal antibodies specific to surface tumor targets with a novel class of highly potent PBD-dimer toxins.PBD dimers do not distort the DNA structure, which makes them invisible to repair mechanisms and allows the cross-links to persist within the DNA.
Unlike earlier generation PBD chemistry, ADC Therapeutics’ proprietary PBD dimers are not a substrate for multi-drug resistance proteins—even in hard-to-treat tumors.Our PBD-dimer technology: A novel approach to hematologic cancers and solid tumors
-
1The antibody drug conjugate (ADC) binds to a specific tumor cell surface antigen and is internalized1,2
-
2The potent pyrrolobenzodiazepine (PBD) dimer toxin is released inside the cell, where it then creates a covalent cross-link between the strands of the DNA double helix2,3
-
3Because these cross-links do not distort the DNA structure, it is hypothesized that they remain invisible to repair mechanisms and can covertly persist to interrupt cell division and cause tumor cell death1-3
Watch an MOA video about our first approved ADC, loncastuximab tesirine-lpyl (2:32)
Our Pipeline
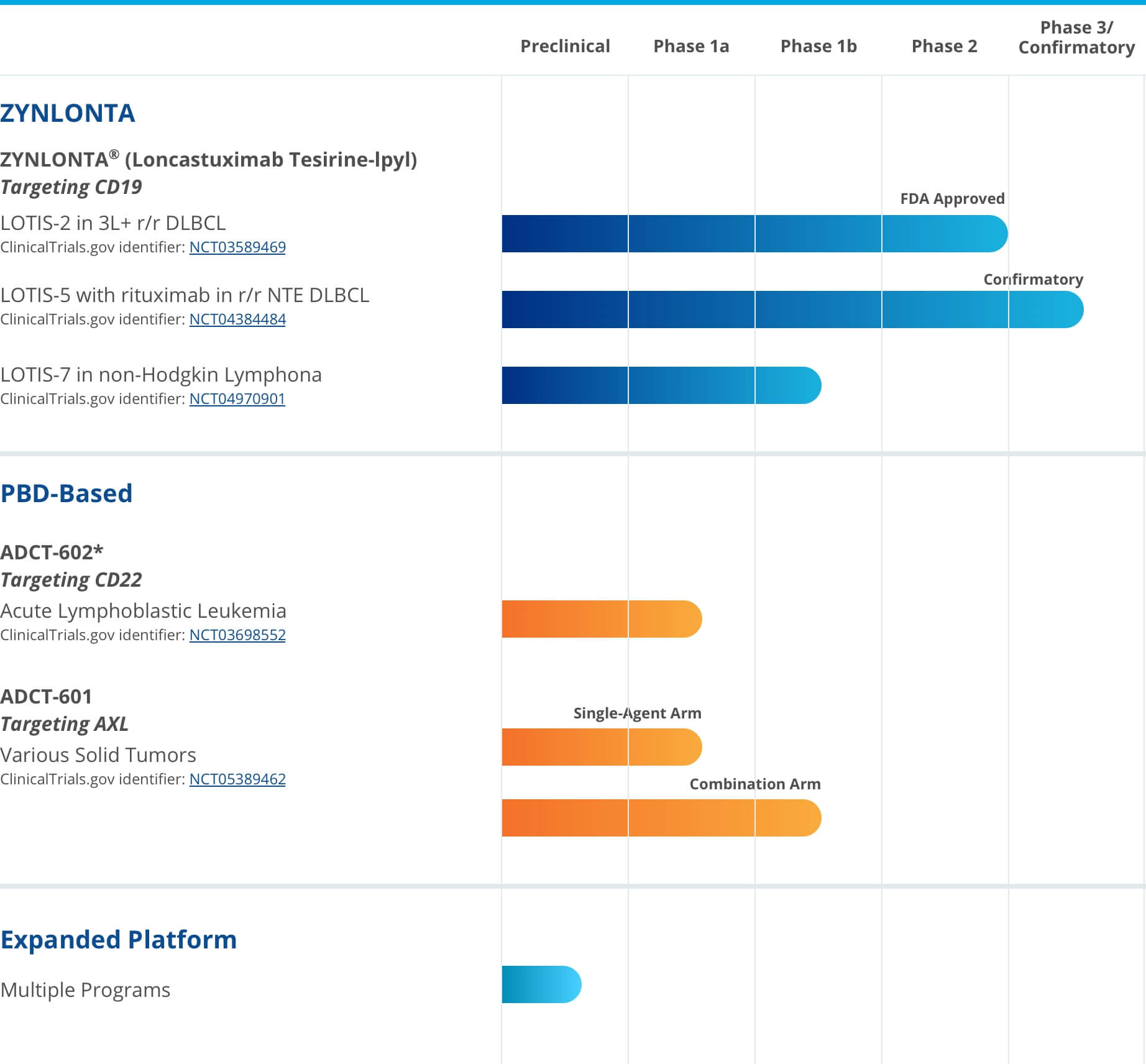
A robust pipeline of investigational ADCs for the treatment of hematological cancers and solid tumorsADCT has multiple PBD-based ADCs in ongoing clinical trials and numerous preclinical ADCs in development.Some of the agents represented in this pipeline chart are investigational. Efficacy and safety have not yet been established.
 * LOTIS-6 trial currently on hold
* LOTIS-6 trial currently on holdExpand each compound to learn more.
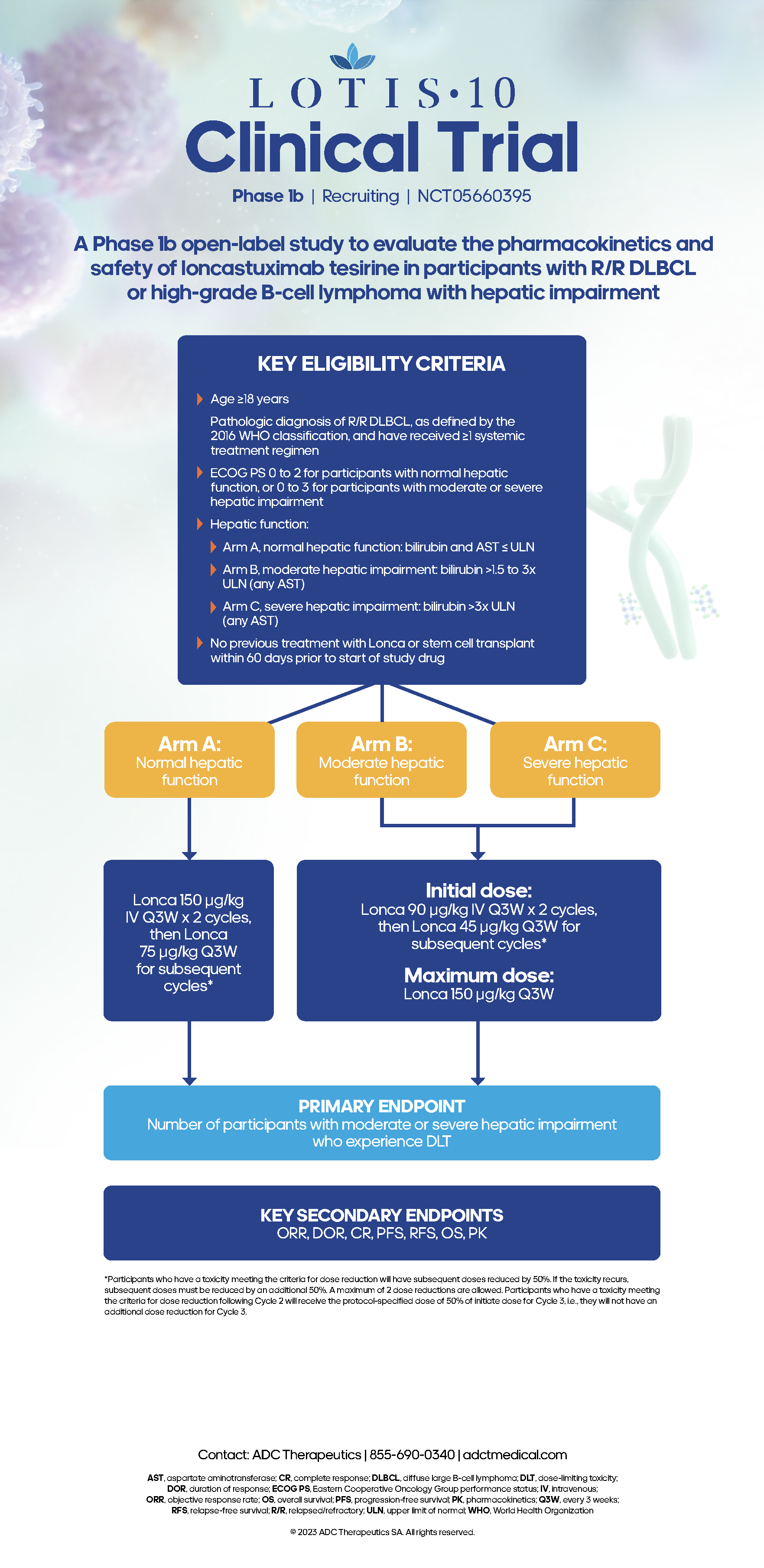
Our Trials
Take a closer look at the LOTIS study designs

Recent Data
Explore the latest research from our scientists
EHA, 2025
View Abstract
EHA, 2025
View Abstract
ICML, 2025
View Abstract
AACR, 2025
View Abstract
AACR, 2025
View Abstract
AACR, 2025
View Abstract
ASH, 2024
Independent Investigator-Initiated Trial
View Abstract
ASH, 2024
Independent Investigator-Initiated Trial
View Abstract
ASH, 2024
Independent Investigator-Initiated Trial
View Abstract
ASH, 2024
Independent Investigator-Initiated Trial
View Abstract
SOHO, 2024
View Abstract
ASCO, 2024
View Abstract
ASCO, 2024
View Abstract
AACR, 2024
View Abstract
SOHO, 2023
View Abstract